IDTechEx discusses three applications for Electronic Skin Patches
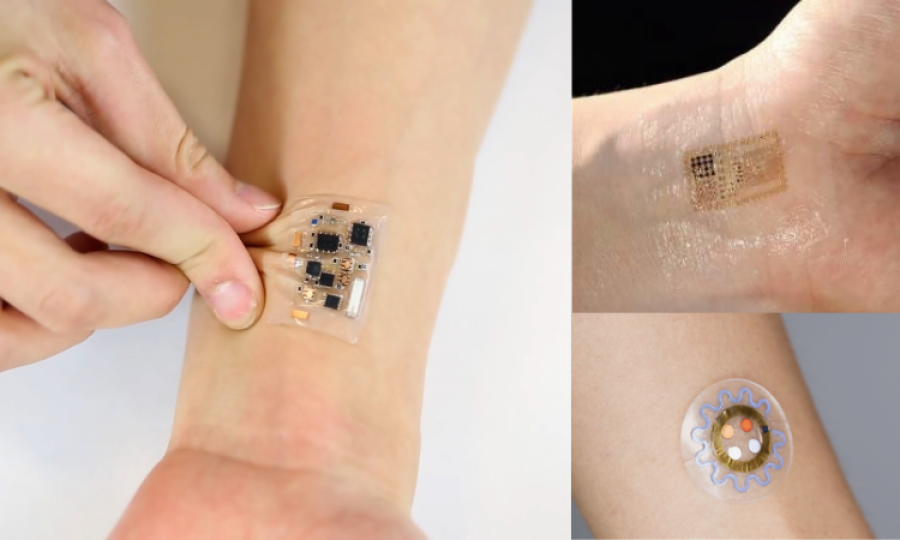
UNITED KINGDOM: The world of healthcare is undergoing significant change. The stress test of the pandemic, a growing global population, and increasing chronic disease prevalence is pushing care to be more efficient and robust.
Technology is facilitating this transformation, and one significant form of wearable technology that is increasingly being adopted by healthcare systems is the electronic skin patch: wearables attached to the skin that can monitor physiology wirelessly and continuously. In their recent report "Electronic Skin Patches 2023-2033", market intelligence firm IDTechEx explores where these emerging devices can add value in different aspects of healthcare.
ECGs: Longer, Lighter, Low-Key
Atrial fibrillation (AF), the most common type of heart rhythm disorder, greatly increases a patient's risk of stroke. Due to its intermittent nature, AF is a very challenging disease to diagnose. To detect AF, doctors monitor the patient's heart rhythm with electrocardiograms (ECG): first, with the 12-lead ECG in the clinician's office, and if more data is needed over a longer period, then with the Holter monitor for a 24-hour period.
Yet, these incumbent monitors are bulky, wired, and uncomfortable for patients, who consequently often pull off the wires and disturb readings. So, patient comfort and adherence to monitoring has been the biggest problem clinicians have been looking for a solution for. This is the key value that skin patches bring. Patients prefer them as a less obtrusive, patient-friendly, and comfortable option.
Adherence to the ECG monitoring program is significantly improved with skin patches than conventional machines, and patients are able and more willing to wear monitors for 48 hours or longer. That has enabled a rapid rise in longer-wearing monitors– extended-wear Holter monitors and Mobile Cardiac Telemetry devices– throughout the 2010s. The success of ECG electronic skin patches has not gone unnoticed, and the 2020s have seen a consolidation in this space: Philips acquired Biotelemetry, Boston Scientific acquired Preventice Solutions, and Baxter acquired Bardy Dx. IDTechEx discusses the industry activity in cardiac rhythm monitoring in their report "Electronic Skin Patches 2023-2033".
Solving Healthcare Staffing Shortages During the Pandemic
In recent years, the major healthcare challenge has been, of course, the COVID-19 pandemic. Pressure from the pandemic has been a catalyst for the rapid adoption of electronic skin patches as part of a greater trend toward remote patient monitoring. The pandemic stretched hospital systems and created staffing shortages.
Electronic skin patches are well-suited to relieve some of this pressure. With wireless communication, automated and continuous monitoring, and battery-outlasting quarantine periods, these devices allow caregivers to monitor several patients in parallel via virtual wards. Several players, like Lifesignals, Vivalink, and Vitalconnect, with remote vital sign monitoring skin patch platforms, saw increased interest in fever monitoring applications during the pandemic.
Beyond the pandemic, remote fever monitoring adds a lot of value for spotting infections early in vulnerable patients. A key example is patients with neutropenia (a reduced white blood cell count) after discharge from chemotherapy, where earlier fever detection via an electronic skin patch worn at home can go a long way to reducing risks and treatment intensity. To learn more about electronic skin patches in vital sign monitoring, please refer to the IDTechEx report "Electronic Skin Patches 2023-2033".
A New Opportunity: Decentralized Clinical Trials
Not only did the pandemic change the healthcare status quo, but it also profoundly affected the pharmaceutical and biotechnology industry. The periods of lockdowns around the world significantly impacted the running of clinical trials, and this triggered affected companies to adopt a model of decentralized clinical trials, a new market opportunity for electronic skin patches.
The rise in decentralized clinical trials is not just a one-time spike as a reaction to the pandemic. Rather, going forwards, users can expect this market opportunity to continue to grow. Decentralized clinical trials bring two key benefits other than those during the pandemic.
First, decentralized clinical trials have a chance to reduce the drop-out rate of trials, often a major cause of failed trials, by removing the challenge of travel to site visits. Second, removing the burden of routine meetings can increase the ability of a clinical trial to reach deprived populations and increase the diversity of the study.
The use of electronic skin patches in decentralized clinical trials is still new, and these devices' overall value has yet to be clearly demonstrated. Nonetheless, expect to see more partnerships between pharmaceutical companies and skin patch players in the coming years.
IDTechEx's research, "Electronic Skin Patches 2023-2033", estimates that the electronic skin patch market will grow to over US$27bn by 2033. This market research report characterizes the markets, technologies, and players in electronic skin patches. With coverage across 13 application areas, historic market data from 2010-2022, and electronic skin patches market forecasts from 2023 to 2033, it is the most comprehensive study compiled for this product area.
Trending
Popular
Sindh pledges vigorous action to prevent poliovirus transmission
-
PMA stresses health equity on World ...
04:08 PM, 9 Apr, 2024 -
Dow University’s new rabies vaccine ...
12:18 PM, 28 Mar, 2024 -
IRD role lauded in advancing ...
02:53 PM, 12 Mar, 2024 -
Over one billion people worldwide ...
09:48 AM, 5 Mar, 2024




